© 2010-2021 by Zaske Software & Technik GmbH
(inter Laboratory Quality Control)
Our product family for automated Quality Control solutions in clinical laboratories
Laboratory testing of patient samples can be a complex procedure, depending on the clinical analysis, a microbiological study or blood bank testing in all areas of the clinical laboratory. Quality Control (QC) is one of the most important effects on laboratory tests – ensuring both precision and accuracy of patient sample results. The integrity of quality control samples is important for both overall quality management and fulfillment of proficiency testing requirements. Addressing QC issues is critical to identifying potential errors in patient outcomes.
If quality control works effectively, it can find errors in a lab’s analytical processes and help correct them before potentially publishing incorrect patient results. Clinical laboratories use documentation management and the integration of a continuous improvement process to streamline the overall quality control process.
Another way to analyze quality control is peer testing and monthly review of QC trends. Clinical laboratories are often involved in clinical laboratory tests (PT) that validate their QC evaluations. Not the result of a single laboratory, but the comparison with a peer group provides security in the validation of QC results. Periodic review of QC results is a common tool for maintaining quality control of patient samples.
For this procedure iLabQC ® is the ideal tool!
iLabQC allows clinical laboratories to evaluate the performance of their assays on the basis of a statistical analysis of QC data generated by the control manufacturer for Chemical, molecular and/or clinical immunochemical analysis devices.
iLabQC collects QC data from different customer controls (from different countries) using the same batch of control material, the same type of analyzer and the same analyte.
iLabQC is independent from any control material manufacturer
iLabQC evaluates your quality data according to different standards such as SFBC, Wetgard etc.
iLabQC captures QC data from almost any device via a hardware or software solution, or receives its QC data via standardized transfer formats (XML).
iLabQC Quality Desktop, a very user-friendly interface.
iLabQC works as a desktop application and offers a variety of evaluation options with the simplest application.
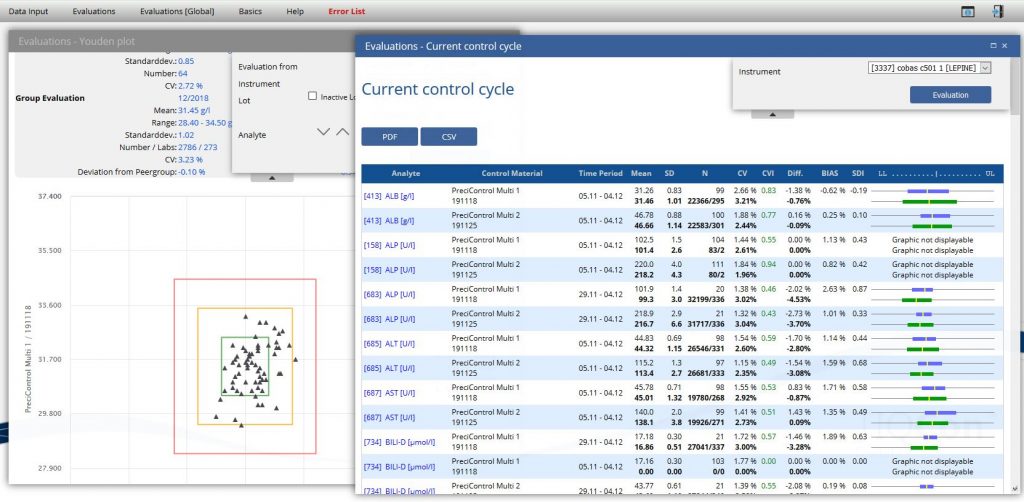
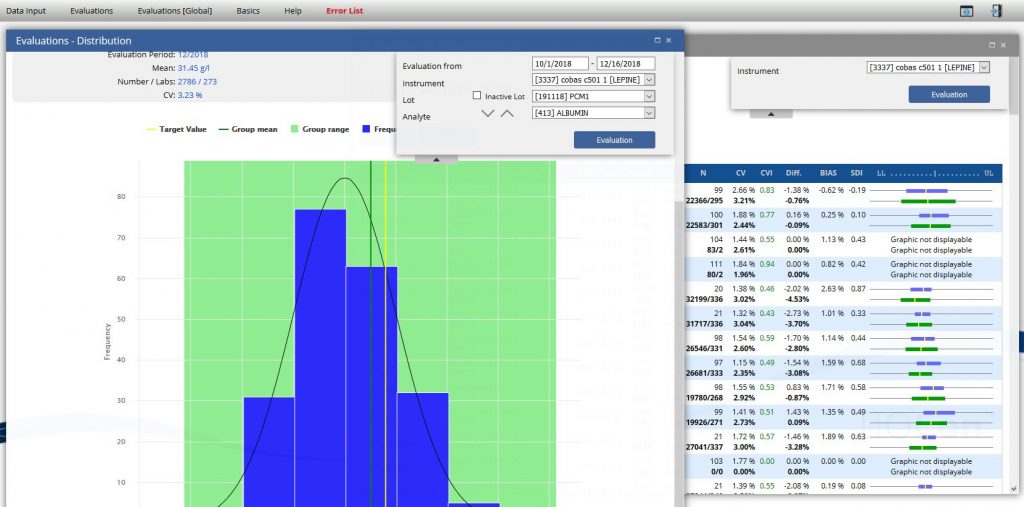
Graphical evaluations and a variety of listed evaltiations Help and support you by checking the quality of your lab.
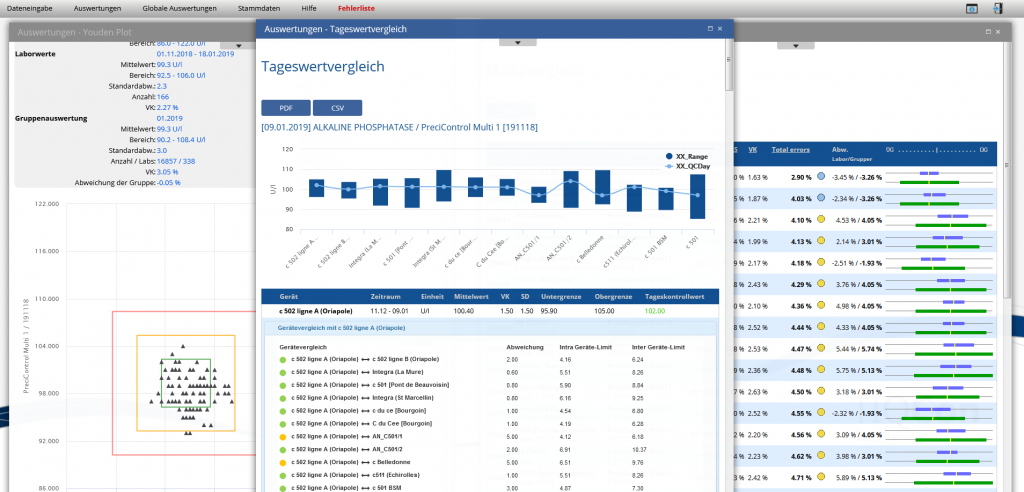
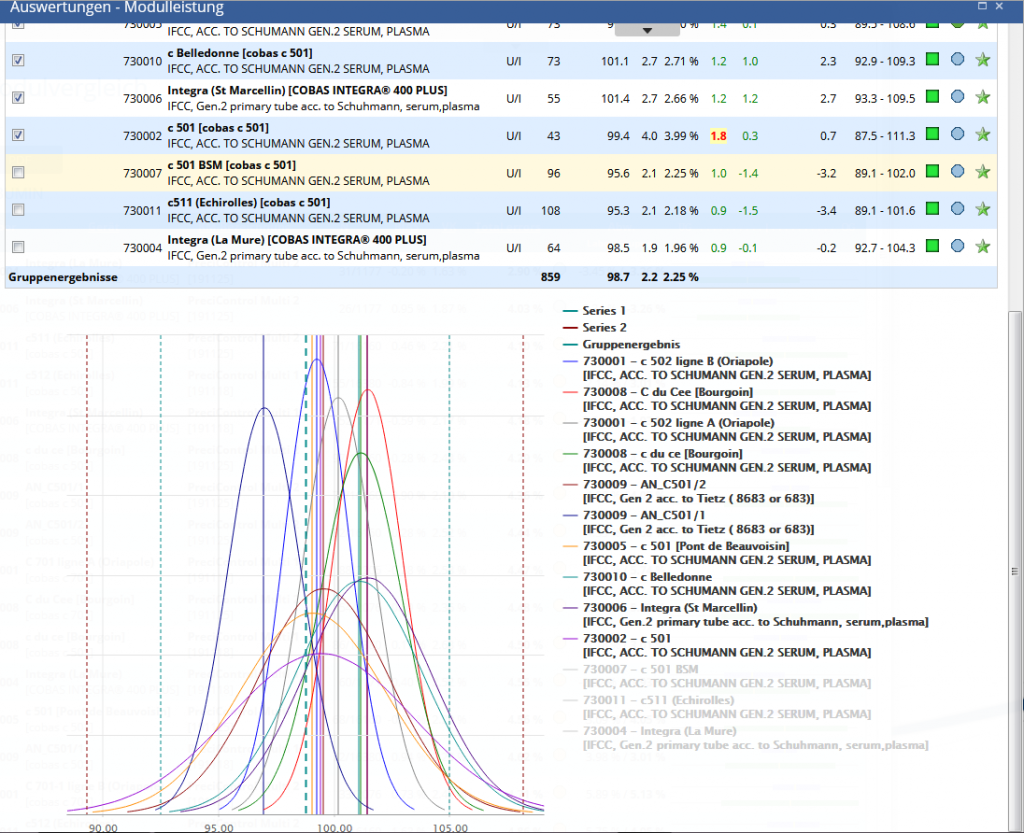
Module / Device Performance compares the performance of a test on different devices in the lab or in the lab chain
iLabQC is the most efficient and user-friendly quality control system for clinical laboratories.
- Compares the internal CQ with the peer group’s cue point.
- Cloud based Solution, connectivity and soft- and hardware data collection solutions.
- Almost any device or analysis protocol can be connected (ASTM,HL7, Behnk etc.).
- Used for clinical chemistry, immunodiagnostics or molecular diagnostics.
Control material lots with their nominal values can be imported and used all over the world. - Can send information and warnings directly via a messenger or push email
- Lab chain management
- Characteristics such as BIAS, CVI, TE and measurement uncertainties are available on the fly
- Laboratory-specific limits for u.a. TE, BIAS and CV max. variation
- Push notifications for critical test results
- Mobile application for status overviews
- Full automated integration integration of QC for supported analyzer
iLabQC is used worldwide as an instrument for accreditation of laboratories and keeps you informed!
For further questions kindly use the following contact : info@zaske-softwaretechnik.com
